Development
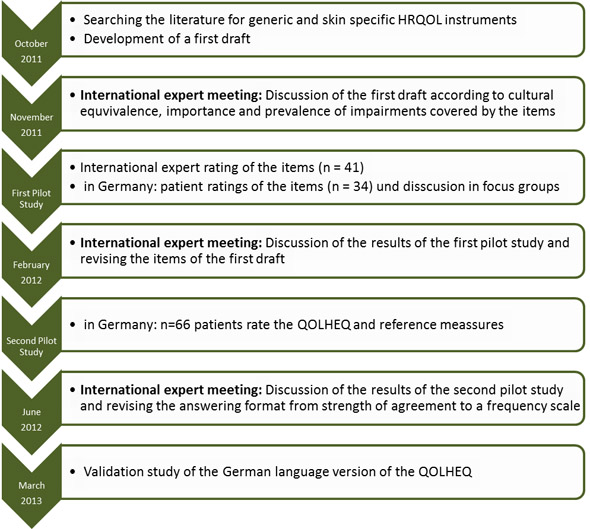
The development process of the QOLHEQ was set according to the guidelines for the development of patient reported outcomes given by the US Food and Drug Administration (FDA). Additionally to those recommendations an international team of experts concerning hand eczema were involved in the development, those experts came from Australia, Denmark, Finland, Japan and Sweden. The following figure gives an overview of the development process. The results of the validation study for the German language version of the QOLHEQ is already published online (Link to article).
 |
